The last couple of weeks has seen some buzz concerning the iQOS, the new Heat-Not-Burn tobacco system from Phillip Morris. From what's publicly available it appears PMI will sell iQOS in the US from early 2017. But what does it mean? Who is it for? What are the wider political and commercial ramifications? What relevance does it have in a market that's already big on vaping?
iQOS Overview
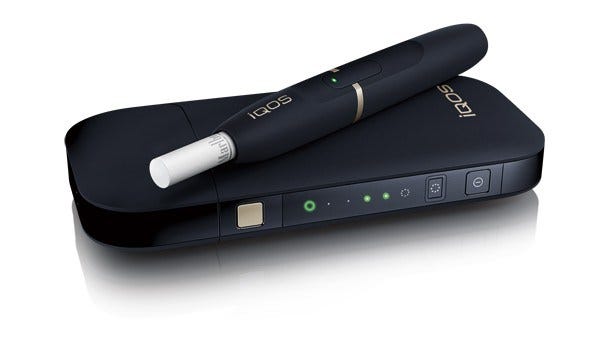
If you've not heard of iQos and HeatSticks, let me get you up to speed quickly. iQOS is a small battery-powered unit into which the user inserts a mini cigarette (HeatStick or "HEET"). iQOS then heats this at a precise temperature. The HeatSticks consist of familiar brands from the PMI/Altria stable such as Marlboro, although exactly how PMI plan on branding them is not yet clear: it appears the HeatSticks themselves are now referred to as "HEETs".
There's no or little combustion from HEETs; instead, the user inhales a tobacco and nicotine-infused vapor rather than smoke. The Heatsticks themselves are a highly engineered product, with a proprietary blend of tobacco/flavourings/excipients that produce a vapor when heated.
iQOS has been available in some countries for some time now, most notably Japan, where PMI claim it has taken around 2.2% (PDF) of the smoking market already. It’s worth pointing out, however, that nicotine-containing vaping products are not available in Japan, so this is in part pent-up demand being satisfied.
Nevertheless, PMIs chief André Calantzopoulos is very bullish about the product, albeit with a slight hedge where he talks about PMI's other platforms. Excepting their e-cig, Mark 10, it's not clear if these other products are yet available.
Why Heat not Burn?
Vapers will be extremely sceptical about these products, largely because of the type of vaping products the tobacco industry has promoted historically. Mark10, Vuse, Logic and Blu are not well-liked products by the vaping user-community. Indeed, some have suggested malfeasance on the part of the majors for promoting products which are not as satisfying as products available in independent channels.
Others point out that the tobacco industry's entry into the vaping market was an inevitability, and that they are not incentivized to develop great products, even as they are incentivized to dominate their existing channels. There's another factor at play too: British tobacco major Imperial Brands owns patents which they claim cover all vaping products currently on the market.
Also, big corporations run slowly whereas innovation in the vaping market has happened at great speed. New products have hit the shelves almost weekly, produced by small, agile players responding to market demands and innovating accordingly. Much of this has been generated by the fantastically competitive manufacturing world of Shenzhen, China, where over 100 factories operate in the vaping space. It's simply not possible for mainstream retailers to be part of this unique market.
In all, then, it's not surprising that the TI isn't doing vape properly. But I don't believe one should discount PMI's overall intentions based on these commercial realities. After all, does anyone believe that the tobacco industry wants to kill its users? To me it's a no-brainer that the tobacco industry will wish to create products that satisfy the following criteria:
- They own the intellectual property.
- The product is inexpensive to make
- But that only they can make it
- The product is easy to use
- and is as similar as possible to their existing products
iQOS checks all these boxes in a way that vape does not and cannot.
I'd go further and say that the commercial opportunity for PMI is quite profound for additional reasons.
Firstly, PM can argue, and already is arguing in some jurisdictions, for lower excise taxes based on the lessened harm compared with cigarettes. Cigarettes have sin taxes added to dissuade consumers from use (and, of course, to create government revenues). The tobacco industry has done very well out of these taxes by using a stalking price-rise strategy. They add a small wholesale price increase at the same time governments apply the larger tax increase; consumers don't notice, and the additional revenues more than cover the losses due to fewer cigarettes sold.
The opportunity for PMI, then, is to undercut their competitors cigarette products, while at the same time realizing more revenues per stick (to use tobacco industry parlance). Indeed, PM makes great hay out of the recent end of cannibalisation of their brands in Japan, and the capture of smokers of competitor brands. I don't imagine Japan Tobacco (the world's 4th largest tobacco company by revenues) are happy!
Is there a place for iQOS?
The thrust of PMI's investor-facing marketing has centred around the notion that smokers don't like vape products; that they need a tobacco product that's as similar as possible to the cigarette. Perhaps unsurprising coming from the tobacco industry, but how much truth is there to it?
Despite being the founder of the world's most influential vaping website, I've never been chauvinistic about vaping. It may or may not prove to be (long term) the biggest disruptor to the cigarette ever seen. It's possible vaping may serve as a precursor to other products that do the job better. Certainly, if you ask most committed vapers whether they'd countenance the use of a product like iQOS, they'd be, at the minimum, skeptical.
Simultaneously, it's crucial to acknowledge that many tobacco users already perform tobacco harm reduction by substituting chew, dip, and snus; products that are vastly less harmful than cigarettes.
My view has always been that smokers should have access to a wide range of nicotine products, and ones that are designed to be attractive to smokers. In other words, not just a small selection of pharmaceuticals aimed at those smokers who've already decided to be patients in need of treatment, but a good range of attractive nicotine products for those who want them.
An abstinence-only culture dominates Tobacco Control in the US, and this culture is fundamentally in conflict with the notion that nicotine can ever be consumed safely by large numbers of people.
And they will almost certainly be thinking about the following.
Replacement smokers?
Without wishing to go too deeply into the culture war between tobacco control and tobacco harm reduction proponents, there's a huge elephant in the room. What is the role of harm-free or harm reduced nicotine?
Most vaping companies are still reasonably small-scale concerns with few shareholders and a short term (but non-the-less committed) vision of their business. The vast majority of vape shops are owner-operated entities. Some are driven purely by the wish to help convert smokers, others are looking for a tobacco company acquisition, but few are planning on creating century-spanning corporations dealing in a sustainable nicotine market. While some may consider these players to be cynical get-rich-quickers, others might take a more nuanced view that it's no bad thing for nicotine companies to plan for (or accept) their future obsolescence in one way or another. It's a horrible irony that it's this sector which the regulations will destroy.
On the other hand, the tobacco industry is one of the world's largest and most profitable, and whose revenues lubricate the global financial system. They have to stay in business and cannot plan for their obsolescence.
One of the current proposals being hotly promoted by US tobacco controllers is to de-nicotinise cigarettes. The 2009 Smoking Act contains provisions that allow the FDA to remove most (but not all) nicotine from cigarettes. The theory is that by doing so, fewer people will become hooked, and those that are hooked will find it easier to quit.
In conversation, I've found proponents of this strategy to be somewhat ambivalent about the role of non-smoked nicotine products such as vaping. Some are absolutely pro the idea of smokers moving onto vaping. Sadly, the follow-on - that smokers should then be allowed to choose from a diverse market in these products - is rarely accepted.
The history of the tobacco industry is replete with examples of highly malfeasant corporate behaviour. Some of these (see the so-called 7-dwarves have left such a huge imprint on our culture that they're somewhat totemic.
The notion of the replacement smoker (PDF) is one such example. Internal correspondence had one RJR executive describing the marketing of cigarettes to adolescent smokers as fulfilling the need to create replacement smokers, thereby perpetuating the corporate tobacco interest.
What does the corporate model of the tobacco industry look like in a harm reduction future?
Well, as I've outlined above, in the short to medium term, very profitable. A small shift from smoked tobacco to heat-not-burn can transfer the "government share" of revenues to the seller through excise changes. Longer-term, it's unclear. Will these products "create replacement iQOSers"? Is nicotine a compound with such utility that more people will choose to use it if safer products are available?
In some scenarios, this could be a new dawn for the tobacco industry. Nicotine could end up being used by more people than currently yet with far fewer health problems associated. Indeed, it's conceivable that these new products are so safe, that it's not possible to even detect any negative health effects in the population.
In other scenarios, the products further renormalise smoking to the extent that replacement smokers decline faster than ever.
Next stage - the politics, the regulations, and the science
PMI has announced that it will apply for Modified Risk Tobacco Product (MRTP) approval with an initial submission to FDA by the end of this year. MRTP is another provision created by the Tobacco Act that allows companies to make a modified risk statement about their product versus cigarettes. First, though, they must persuade the FDA that such a statement is an accurate assessment of scientific fact.
To date, only one company has attempted to gain MRTP approval. Swedish Match spent an estimated $50million on research and submitted over 100k pages of scientific testimonial for their General Snus range of oral tobacco products. Despite overwhelming evidence that (all) snus is vastly safer than smoking the application was rejected by the approval committee (TPSAC) and the process is still ongoing. So far, the politics have won over the science.
Gaining MRTP status might sound like a huge effort for not much payoff: the ability to state something that's self-evidently true. But Harm Reduction is, fundamentally, a communication intervention, and currently, the law forbids this communication. The small informational concession from a successful MRTP application could see a sea-change in smoker behavior at the macro level. The question is whether the politics is so poisoned that it will ever be allowed to happen.
PMI: It's almost as if they planned this!
One thing's for sure, though: no-one has prepared for this moment better. For over a decade PM has been conducting research, developing novel methods for assessing health effects and refining their iQOS platform. In all, over $3bn has been spent on this project. PMI's MRTP submission to the FDA is said to contain over two million pages, and the timing here is insanely good; so good it's almost like they convened the forces of the universe themselves:
In August this year, new deeming regulations came into play. FDA now deems vaping products and other novel non-smoked tobacco products to be Tobacco Products and to fall under their regulatory jurisdiction. No new products are allowed on the market from August 8th, and all the goods currently on the market may remain for two years while their manufacturers apply for PMTA.
[Edit - since publishing, I have been informed that the following paragraph is based on incorrect information, and that PMI will not be marketing iQOS until such a time as they have received premarket authorization, possibly some time in 2017/2018]
And guess what? According to PMI, iQOS was sold in 10 US cities on August 8th this year. They are now free to sell their product for two years while anyone else who was not on the market must gain pre-approval.
The PMTA process is so onerous that few brands are expected to make it through. There's a huge question mark as to what is on the market in two to three year's time to satisfy existing consumer demand. PMI will be there waiting.
BAT, Reynolds and JTI?
So far, not a peep has been heard from other tobacco companies on their plans for new tobacco products in the US market. BAT are making moves to acquire Reynolds American, facilitated by economic conditions caused by Brexit. BAT has unique research capabilities and R&D, and already has a technology sharing agreement with Reynolds. It's conceivable that BAT or Reynolds also grandfathered some novel products on August 8th, but so far no public statements have been made to this effect.
The regulatory advantage to PMI, according to current information, is that they have a head start which could prove to be a critical window in which they can seek to dominate. Some business theorists would state that first-mover advantage is a myth, but I'm not sure this applies here: Heat-not-Burn isnot new whereas this regulatory paradigm certainly is.
The long term?
So, the long-term question is: can PM make such inroads with their new technology as to be the disruptors and long-term winners in a market that has been forged by the emergence of vaping?
Well, this takes us back to the question posed at the beginning. What is the market for iQOS in a nation that already has vape?
I'll leave you with a few quotes from people I know who've used iQOS.
I'm worried about using it because it's too similar to smoking and I worry that I'll go back to smoking
I'm back on vaping because I can't get hold of the damned things
I went back to vaping because I was getting through 3 packs per day and it was too expensive
Nice product - fits well inside my pocket, but it's not as satisfying at the real thing
So, a mixed bag so far. Prediction's a mugs game - but I'd rather see iQOS emerging as part of the story, and not the whole story.





Leave a comment
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.